Hybridization is a cornerstone of chemical bonding that can be likened to a metamorphosis—a transformation where different entities converge to form something uniquely functional. In this elaborate dance of atomic structure, lone pairs emerge as enigmatic partners, influencing the hybridization of molecules in profound ways. Understanding the nuances of hybridization, particularly in the presence of lone pairs, reveals the intricacies of molecular geometry and reactivity.
At its essence, hybridization involves the blending of atomic orbitals to create new, equivalent orbitals capable of accommodating bonded electrons. These are the resultant formations that bestow molecules with distinct geometrical shapes and electronic configurations. However, the advent of lone pairs, those elusive electron pairs existing in isolation, adds another layer of complexity and intrigue. Much like solitary dancers on a grand stage, lone pairs can significantly alter the dynamics of bonding, influencing not only hybridization but also the overall persona of a molecule.
To grasp the phenomena of hybridization with lone pairs, one must first comprehend the fundamental types of hybridization: sp, sp2, and sp3. Each type correlates with specific geometrical shapes governed by the number of electron clouds surrounding a central atom. In the case of sp hybridization, where one s and one p orbital merge, the resulting linear configuration finds its place in molecules like acetylene (C2H2). Here, lone pairs are notably absent, allowing for a precise linearity—an unyielding structure reminiscent of perfectly aligned arrows.
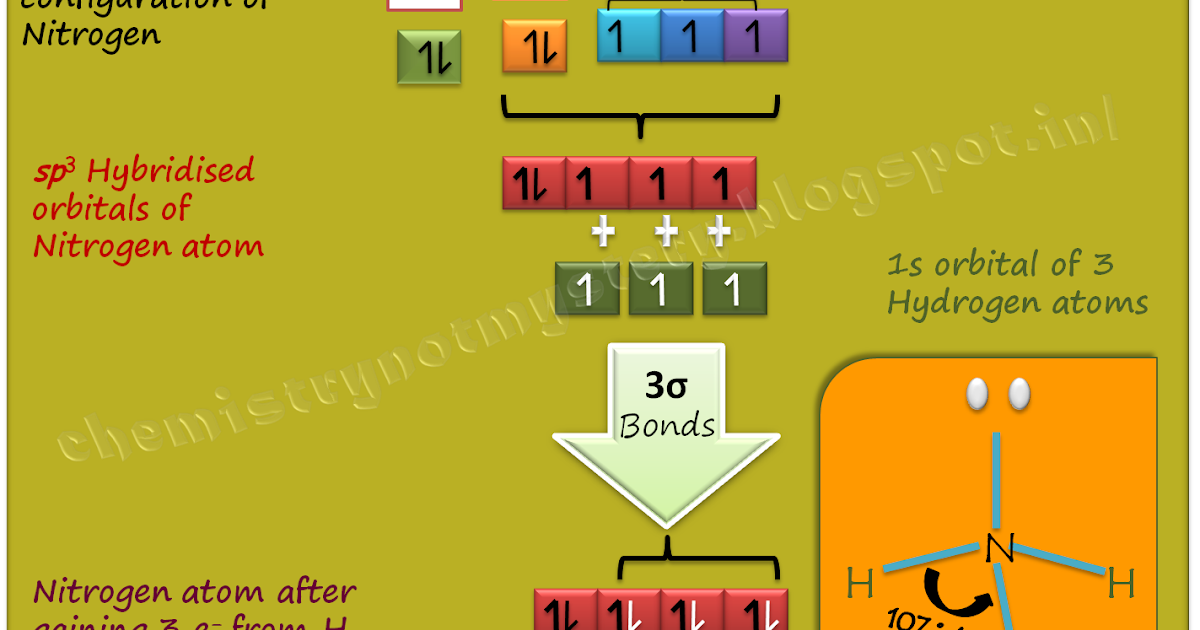
In contrast, the sp2 hybridization introduces a more complex choreography. In this scenario, one s and two p orbitals hybridize to establish a trigonal planar shape observable in ethylene (C2H4). When lone pairs make an entrance, they can skew the perceived balance of the molecular geometry. A quintessential example is ammonia (NH3), where the nitrogen atom engages in sp3 hybridization. The three bonding pairs and one lone pair yield a trigonal pyramidal geometry, aptly illustrating how a single solitary partner can tilt the entire structure, much like a skilled juggler who inadvertently shifts the focus by bringing in an additional ball.
The nature of lone pairs is multifaceted; they do not merely occupy space. Rather, they exert influences over the molecules they inhabit. Lone pairs, residing in their own private orbitals, are determined to repel any nearby electron clouds—either bonded or otherwise. This repulsion is often greater than that experienced between bonding pairs, which leads to distorted geometries. In the realm of sp3 hybridization, for example, the tetrahedral angles (ideally 109.5 degrees) are compressed when lone pairs are brought into the mix, leading to an observable change in bond angles, as seen in water (H2O) where the angle measures approximately 104.5 degrees. Here, the lone pair’s insistence to claim space adds to the molecular’s own distinctive identity.
The implications of hybridization with lone pairs extend beyond mere shape alteration. They delve into the realm of reactivity and interaction with other molecules. Polar and nonpolar characteristics are often dictated by the presence and orientation of lone pairs. In water, the lone pairs create a net dipole moment, establishing water as an excellent solvent and a quintessential medium for life processes. This crucial contribution showcases how hybridization, flavored with the distinctiveness of lone pairs, serves as a conduit through which essential biochemical reactions occur. It emphasizes the interconnectedness of structure and function at the molecular level. In this way, each hybridized molecule is akin to a symphony, where each atomic partnership, including those lone pairs, contributes to a cohesive and functional whole.
As we explore further, consider the resonance structures that emerge when lone pairs take center stage. Some molecules can exist in multiple forms by transferring lone pairs among atoms, allowing for a richer tapestry of reactivity and stabilization. This phenomenon adds depth to the understanding of how lone pairs may govern a molecule’s interactions with external entities, especially in biochemical environments where precision is paramount. They orchestrate a delicate balance, allowing molecules to adapt and engage in myriad chemical reactions while maintaining their integrity.
Moreover, hybridization with lone pairs is also influential in predicting molecular polarity. Molecules with asymmetrical distributions of electron density due to the presence of lone pairs result in polar molecules. Consider hydrochloric acid (HCl)—the lone pair on the chlorine atom creates a dipole moment that facilitates its interactions in aqueous solutions, enhancing its reactivity. This facet underscores a critical point: the artistic potential of lone pairs goes beyond aesthetics; they actively contribute to chemical landscapes.
In summary, the dance of hybridization interwoven with the enigmatic presence of lone pairs weaves a narrative that extends deep into the heart of chemistry. Each partnership influences molecular behavior and characteristics, guiding the paths of biochemical reactions and structural determinations. The analogy of a stage performance illuminates this compelling interplay, where each actor—be it an atom or a lone pair—has a significant role in shaping the outcome. Embracing this complexity in molecular interactions not only enriches our comprehension of chemistry but becomes imperative in applications like drug design, materials science, and environmental chemistry. Understanding these interactions not only paves the way for fostering innovations but also helps in addressing the exigent challenges of climate change by allowing scientists to engineer more effective, sustainable solutions. Thus, in the intricate dance of molecules, hybridization with lone pairs serves as a crucial focal point guiding the evolution of scientific thought and practical applications.