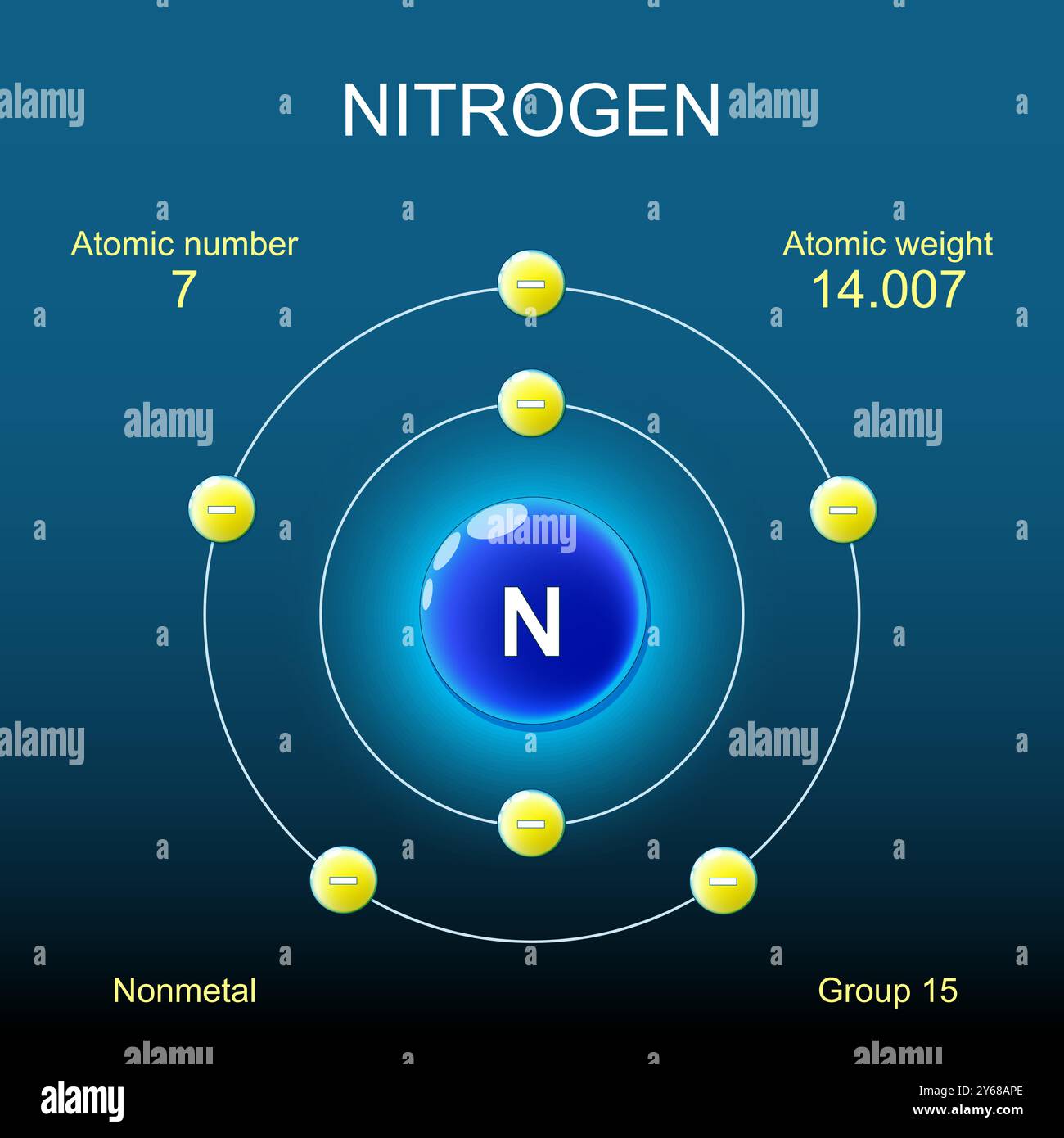
Nitrogen, a vital element in both biological and chemical processes, exhibits intriguing properties due to its unique hybridization. This phenomenon affects how nitrogen atoms bond with other atoms, shaping the very essence of life as we know it. But what exactly is nitrogen hybridization, and how does it influence the myriad compounds we rely on daily? Let’s delve into the multifaceted world of nitrogen hybridization, exploring its intricacies and posing a playful question: Can the arrangement of nitrogen’s electrons determine the fate of complex life forms on Earth?
To begin, nitrogen resides in group 15 of the periodic table, possessing five valence electrons. Its electronic configuration provides a fertile ground for hybridization, a concept that describes the mixing of atomic orbitals to create new hybrid orbitals. These hybrid orbitals facilitate more efficient bonding with surrounding atoms, resulting in diverse molecular structures. The two most prevalent forms of nitrogen hybridization are sp3 and sp2. Each configuration contributes distinct properties to nitrogen compounds.
The sp3 hybridization occurs when one s and three p orbitals combine, yielding four equivalent sp3 hybrid orbitals. In this arrangement, nitrogen forms molecules such as ammonia (NH3), where the nitrogen atom covalently bonds with three hydrogen atoms. The shape of the ammonia molecule adopts a trigonal pyramidal geometry, lending it unique physical and chemical properties, such as its ability to dissolve in water and its role as a nutrient source in agriculture.
On the other hand, sp2 hybridization results from the mixing of one s and two p orbitals, creating three equivalent sp2 hybrid orbitals. This hybridization is particularly significant in organic nitrogen compounds, like imines (R–C=N–R’) or nitriles (R–C≡N). In these compounds, nitrogen often engages in double bonds, altering its reactivity and stability. The planar configuration of sp2 hybridized nitrogen atoms lends itself to electronic delocalization, which can influence the compound’s properties and behavior in various chemical environments.
Now, one may ponder—how does the hybridization of nitrogen affect its role in the biosphere? The answer lies in understanding how nitrogen compounds cycle through ecosystems. Nitrogen is an essential nutrient for plants, predominantly existing in the form of ammonia or nitrates in the soil. When plants absorb these forms of nitrogen, they utilize them to synthesize amino acids, proteins, and nucleic acids, vital components for cellular structure and function. Alas, here arises a challenge: industrial agriculture, which employs nitrogen fertilizers, incentivizes hybridization pathways that may not align with natural processes, potentially leading to environmental ramifications.
Moreover, nitrogen enters the atmosphere through various means, including biological fixation, where specific bacteria convert atmospheric nitrogen gas (N2) into ammonia via the Haber-Bosch process. This process, while revolutionary for agriculture, also warrants scrutiny due to its significant energy demands, largely derived from fossil fuels. The interplay between nitrogen hybridization and biospheric processes is hence not merely a question of chemistry; it intertwines with ecological balances and climate change considerations.
Interestingly, the nitrogen cycle comprises several stages, each intricately linked to the hybridization of nitrogen. After nitrogen fixation, plants assimilate organic nitrogen, which is utilized by herbivores and subsequently transferred through the food chain. When organisms die, decomposition returns nitrogen to the soil, reinitiating the cycle. As such, hybridization plays a pivotal role in facilitating various nitrogen compounds throughout these biogeochemical pathways, showcasing the element’s versatility.
The hybridization phenomena also extend to the realm of atmospheric science. Nitrogen oxides (NOx), produced from combustion processes, serve as significant contributors to air pollution and climate change. The sp2 hybridization present in nitrogen oxides allows for a versatile bond structure, enabling these compounds to partake in complex atmospheric reactions. These processes can lead to the formation of secondary pollutants, emphasizing the need for stringent regulations to manage emissions effectively.
As the world grapples with climate challenges, understanding nitrogen hybridization offers a critical juncture between chemistry and environmental stewardship. The question arises—what innovative strategies could scientists employ to harmonize nitrogen utilization in agriculture with sustainability? Perhaps the answer lies in biotechnological advancements or the adoption of organic farming practices that emphasize natural nitrogen cycling.
Additionally, the concept of nitrogen hybridization invites further exploration into its applications in energy production. As researchers investigate alternative fuels, the potential for harnessing nitrogen compounds could open new avenues for sustainable energy solutions. This innovation may not only help mitigate atmospheric greenhouse gases but also reduce reliance on nonrenewable resources.
In closing, nitrogen hybridization is far more than a mere academic concept; it profoundly influences ecological dynamics, agricultural practices, and climate policies. Understanding its significance is crucial in addressing the environmental challenges we face today. As stewards of the Earth, exploring the intricate tapestry of nitrogen’s role in our ecosystems may illuminate pathways toward a more sustainable future. The playful question remains: can we better manage nitrogen to foster a thriving planet for generations to come? This inquiry underscores the essence of scientific inquiry: the constant pursuit of knowledge to inspire action and solutions for our world’s pressing issues.