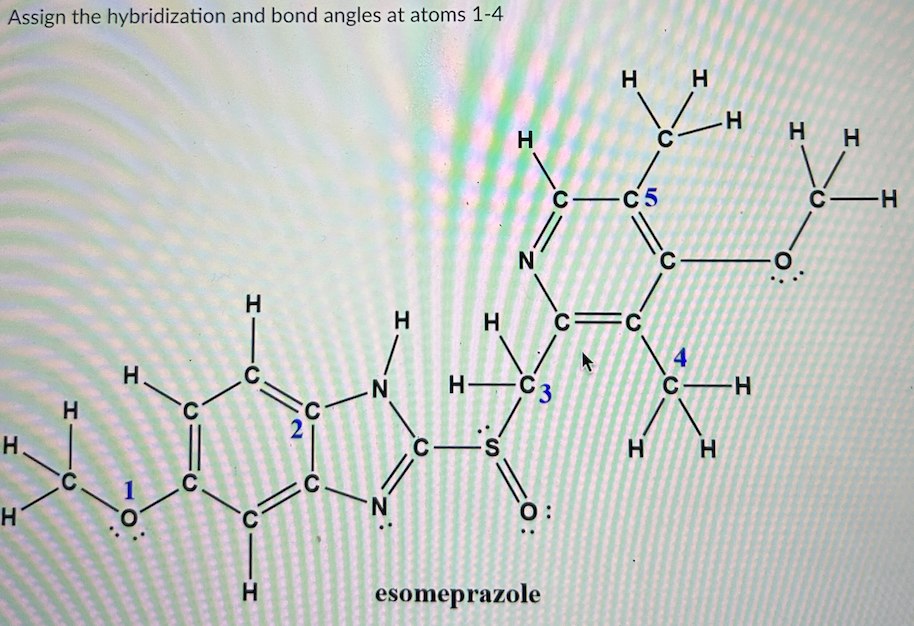
Hybridization and bond angles are fundamental concepts in the field of chemistry, intricately entwined with the understanding of molecular geometry and the behavior of atoms in chemical compounds. The study of how atomic orbitals combine to form new, hybrid orbitals is pivotal in comprehending the spatial arrangements of atoms, which ultimately dictate the properties of molecules.
At its core, hybridization refers to the mixing of atomic orbitals from the same atom to create new orbitals that possess characteristics suitable for forming bonds in a molecule. This process illustrates the adaptability of atomic structures in the quest for stability through bonding. For instance, in carbon, the combination of one s orbital and three p orbitals results in four equivalent sp³ hybrid orbitals, each directed towards the corners of a tetrahedron.
The concept of bond angles arises from the spatial orientation of these hybrid orbitals. In simple terms, bond angles are the angles formed between two adjacent bonds that stem from a central atom. They provide crucial insight into the three-dimensional arrangement of atoms within a molecule. In a tetrahedral configuration, common for molecules like methane (CH₄), the bond angles measure approximately 109.5 degrees, which is optimal for minimizing electron-electron repulsion among the surrounding electron clouds.
Delving deeper into hybridization, we find several types that are categorized based on the number of atomic orbitals involved. sp, sp², and sp³ hybridizations represent different scenarios where carbon can form four bonds through various spatial orientations. sp hybridization, for example, occurs when an s orbital combines with one p orbital, leading to two linear hybrid orbitals at a bond angle of 180 degrees as seen in acetylene (C₂H₂).
Conversely, in sp² hybridization, one s orbital mixes with two p orbitals, producing three hybrid orbitals that are coplanar, with bond angles of approximately 120 degrees. This configuration can be observed in ethylene (C₂H₄), where the double bond introduces a planar structure that facilitates resonance and enhances its reactivity. The presence of pi bonds, generated from unhybridized p orbitals, contributes to the stability and selectivity of these molecular arrangements.
Each type of hybridization conveys a distinct preference for molecular shape. The VSEPR (Valence Shell Electron Pair Repulsion) theory complements hybridization by elaborating on how the presence of lone pairs affects these angles. Lone pairs occupy more space than bonded pairs, causing bond angles to deviate from their predicted values. Ammonia (NH₃), for example—while sp³ hybridized with expected angles of 109.5 degrees—exhibits bond angles of about 107 degrees due to the repulsive force exerted by the lone pair on the nitrogen atom.
When considering hybridization and bond angles, it is critical to recognize how these concepts are not merely academic curiosities but serve as the underlying principles for explaining the behavior of organic molecules and biochemical pathways. This understanding is foundational for applications spanning various disciplines—from pharmaceuticals to materials science—where molecular shape and reactivity are paramount.
The thematic relationship between hybridization and molecular geometry also fosters a deeper appreciation for the aesthetics of chemical structures. Even beyond their functionality, the shapes that emerge from hybridization tell a story of natural design. The elegant, interconnected architecture of molecules exemplifies the delicate balance of forces at play, revealing a complex tapestry woven throughout nature.
Moreover, the ever-increasing knowledge of hybridization opens pathways to innovations in sustainable energy solutions and environmental stewardship. For example, harnessing the unique properties of specific molecular shapes can lead to the development of advanced materials for solar energy conversion or more efficient catalysts for reducing environmental pollutants. Here, chemistry’s artistry intersects with practical applications that resonate with the values of conservation and sustainable practices.
As we move towards an era of heightened environmental awareness and technological advancement, the implications of hybridization and bond angles extend beyond mere scientific curiosity. They emerge as critical players in designing molecules that could mitigate climate change or contribute to the creation of greener technologies. In this context, the significance of understanding bond angles and hybridization techniques becomes clearer, manifesting as a linchpin in the broader quest for a more sustainable future.
In conclusion, hybridization and bond angles are not just foundational concepts in chemistry; they represent the union of science and artistry, illuminating the intricacies of molecular behavior. The ongoing exploration of these concepts uncovers connections that transcend the laboratory, influencing myriad applications that align with the modern imperative of sustainability. As we delve into the molecular world, we reveal the underlying elegance that governs chemical interactions, urging us to recognize and harness this knowledge for a more harmonious relationship with our environment.