In the intricate realm of chemistry, where atoms dance in a delicate waltz of attraction and repulsion, the concept of hybridization emerges as a compelling metaphor for creating harmony among seemingly disparate entities. Just as an orchestra combines various instruments to produce a melodious symphony, atoms hybridize to form new, stable bonds that enable the construction of the wondrous molecular architecture we observe in nature. This blending of atomic orbitals not only elucidates the complexities of chemical bonding but also underpins the grand narrative of matter in our universe.
At its core, hybridization refers to the mixing of atomic orbitals to generate new hybrid orbitals, which can engage in bonding arrangements that maximize stability and minimize energy. These hybrid orbitals are not mere random combinations; they are sophisticated constructs designed to adapt to the geometrical requirements of molecular formation. This process is akin to a master chef who carefully selects and combines ingredients to craft a unique dish, each component serving a purpose that contributes to the whole.
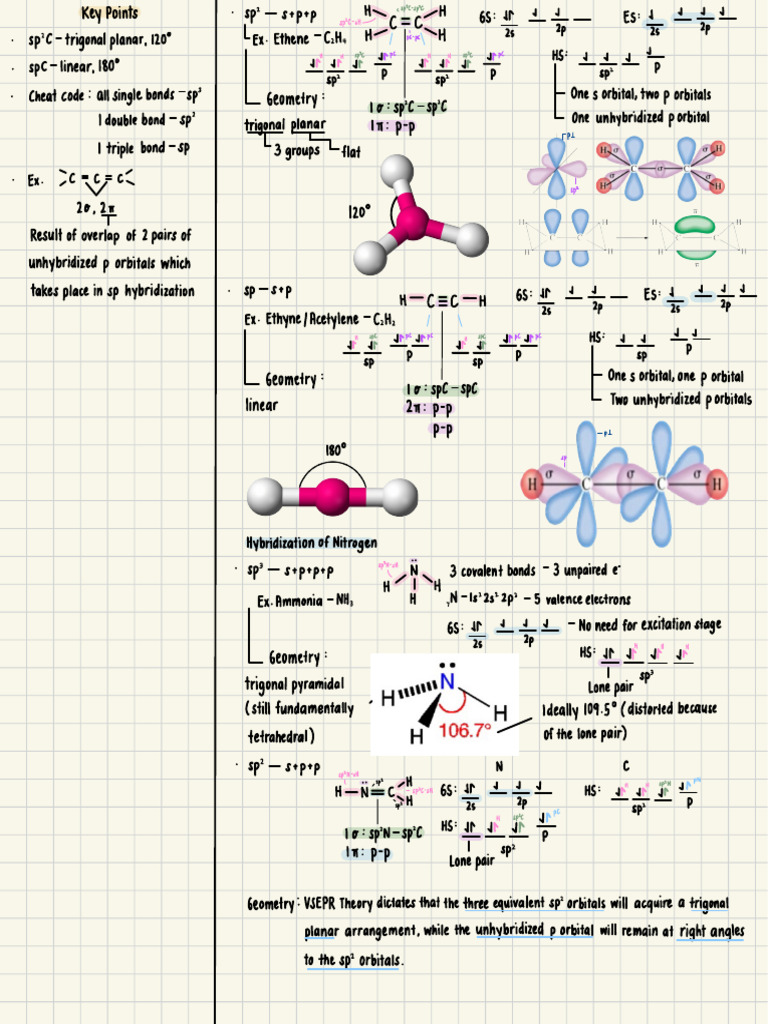
Consider the carbon atom, a pivotal player in the story of organic chemistry. Carbon’s ability to hybridize is a key factor in its versatility, making it the backbone of countless organic compounds. When carbon bonds with other elements, it often undergoes sp3, sp2, or sp hybridization. Each type of hybridization corresponds to a distinct geometry and bonding capability, ultimately influencing the characteristics of the resulting molecules.
In sp3 hybridization, the carbon atom merges one s orbital with three p orbitals, yielding four equivalent sp3 hybrid orbitals that orient themselves in a tetrahedral configuration. The classic example here is the methane (CH4) molecule, where the carbon atom forms four sigma bonds with hydrogen atoms. Visually, one can imagine a tetrahedron, its vertices representing the hydrogen nuclei stretched out from a central carbon nucleus. This arrangement minimizes electron pair repulsion and fosters a stable molecular shape.
The simplicity of methane belies a deeper truth: hybridization is not just a mere bond-forming technique but a fundamental principle that permits the diverse tableau of organic compounds. In sp2 hybridization, the carbon atom engages in a different form of creative expression. Here, one s orbital merges with two p orbitals to form three sp2 hybrid orbitals, which are arranged in a trigonal planar configuration. This allows for efficient bonding in compounds such as ethylene (C2H4), where the carbon atoms are able to form a double bond through the overlap of unhybridized p orbitals. The resulting structure is planar, granting it unique properties that make it suitable for various applications, including in the synthesis of plastics and pharmaceuticals.
Taking a step further, we encounter sp hybridization, where one s and one p orbital conjoin to produce two linear sp hybrid orbitals. This configuration dictates a linear geometry, as seen in acetylene (C2H2), a compound characterized by its triple bond. Here, the carbon atoms exhibit an elegant linear alignment, resembling a taut string ready to resonate at the slightest disturbance. The presence of two pi bonds alongside the sigma bond in acetylene renders it a highly energetic compound, valued not only for its fleeting bursts of combustion but also for its role in the synthesis of various organic compounds.
Hybridization schemes extend beyond carbon offerings, infiltrating other elements in the periodic table and showcasing remarkable versatility. For instance, nitrogen utilizes sp3 hybridization in ammonia (NH3), while the unique bonding characteristics of phosphorus and sulfur illustrate the expansive potential of hybridization. When sulfur forms SF6, it adopts an octahedral structure, utilizing sp3d2 hybridization, transcending the standard octet rule and showcasing the adaptability of these atomic configurations.
The beauty of hybridization is further illuminated by its integral role in the field of molecular symmetry. The symmetry of a molecule often dictates its reactivity and overall properties. Hybridization generates orbital arrangements that comply with the principles of symmetry, granting insights into the molecular geometry and the potential for external transformations. This elevates the importance of hybridization from a foundational chemical concept to a guiding principle in the synthesis and design of novel compounds.
In modern chemistry, the implications of hybridization echo far beyond the confines of laboratories. As scientists endeavor to harness the power of molecular interactions for innovation, understanding hybridization lays the groundwork for advances in material sciences, pharmaceuticals, and energy solutions. By shaping bonds at the atomic level, hybridization enables the creation of new materials that serve as sustainable alternatives, a necessity in the face of climate change challenges.
Ultimately, the narrative woven through hybridization encapsulates the essence of chemistry as an art form—an integration of structure, function, and beauty. It invites us to view molecular interactions not merely as chemical equations but as dynamic relationships that echo throughout our universe. As we continue to explore the depths of hybridization, we unveil a tapestry rich with potential, innovation, and the promise of a sustainable future. In this world of atoms, where bonds represent connections not only between elements but also between ideas and solutions, hybridization stands as a testament to the ingenious adaptability of nature.