Hybridization in chemistry is a pivotal concept that elucidates the formation of molecular structures through the merging of atomic orbitals. This phenomenon is essential for understanding the diverse array of molecular geometries and bonding characteristics exhibited by atoms when they form compounds. In this discussion, we will explore various types and examples of hybridization, delving into sp, sp², sp³, and other less common hybridization forms.
To comprehend hybridization, one must first appreciate its grounding in quantum mechanics and atomic structure. Electrons reside in orbitals, which can be categorized as s, p, d, and f types. Each of these orbitals possesses distinct shapes and orientations, dictating how atoms will surround themselves with electrons during bonding. Hybridization merges these atomic orbitals to create new, equivalent hybrid orbitals conducive to achieving optimal bond angles, thereby promoting stability in molecular structures.
One of the simplest forms of hybridization is sp hybridization. This type occurs when one s orbital and one p orbital combine to form two equivalent sp hybrid orbitals. The resultant structure has a linear geometry characterized by a bond angle of 180 degrees. A quintessential example of sp hybridization is found in acetylene (C₂H₂), where each carbon atom utilizes sp hybridization to form a triple bond. The linear nature of the molecule is paramount to its reactivity and properties, a common theme seen in molecules exhibiting this hybridization.
Transitioning to sp² hybridization, we observe a more complex arrangement. Here, one s orbital and two p orbitals amalgamate to form three sp² hybrid orbitals, maintaining a trigonal planar geometry with bond angles of approximately 120 degrees. This hybridization pattern is prevalent in molecules such as ethylene (C₂H₄). Each carbon atom in ethylene utilizes sp² hybridization to form a double bond with another carbon atom while also engaging in sigma bonding with hydrogen atoms. The presence of this double bond introduces resonance and affects the molecule’s reactivity, distinguishing it from its aliphatic counterpart, ethane.
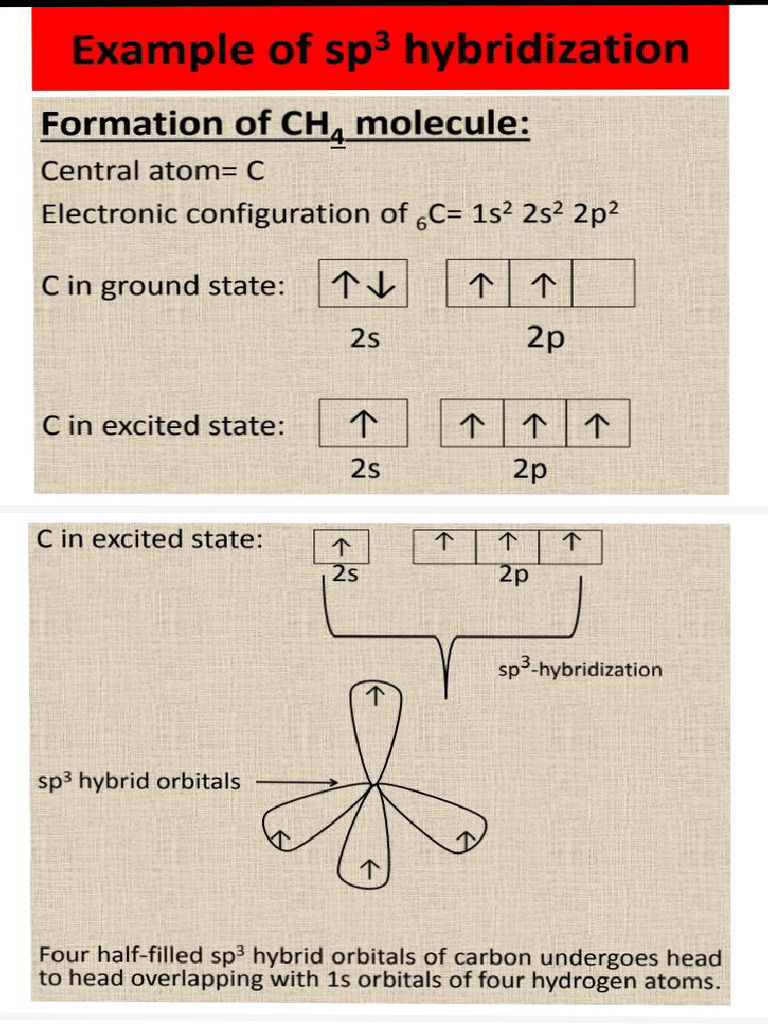
Furthermore, sp³ hybridization emerges when one s orbital combines with three p orbitals to create four equivalent sp³ hybrid orbitals. This configuration is characteristic of a tetrahedral geometry with bond angles around 109.5 degrees. Methane (CH₄) serves as the archetype for sp³ hybridization. In methane, the carbon atom’s four sp³ hybrid orbitals form a single sigma bond with hydrogen atoms, exemplifying the principles of hybridization through its symmetrical tetrahedral shape. This arrangement minimizes electron repulsion, leading to the stability of organic compounds.
As we delve deeper into hybridization, it becomes crucial to consider additional hybridization types that emerge under specific circumstances. One such type is sp³d hybridization, which occurs when one s orbital, three p orbitals, and one d orbital combine. This hybridization is observed in molecules with trigonal bipyramidal geometries, such as phosphorus pentachloride (PCl₅). Here, the phosphorus atom employs sp³d hybridization to bond with five chlorine atoms, establishing unique axial and equatorial positions, thus influencing the overall molecular polarity.
In addition, sp³d² hybridization represents another advanced form of hybridization involving one s orbital, three p orbitals, and two d orbitals. This is typically found in octahedral molecules, one classic example being sulfur hexafluoride (SF₆). The octahedral shape facilitates uniform distribution of bond angles at 90 degrees, thus enhancing the molecular stability. It is essential to recognize that such hybridization types facilitate the formation of complex molecules necessary for various biologically and industrially relevant processes.
The application of hybridization extends beyond mere academic inquiry; it has tangible implications in drug design and material science. Understanding hybridization enables chemists to predict the behavior of molecules in reactions and leads to the development of novel compounds with desired properties. Moreover, hybridization informs the design of catalysts, which are crucial in accelerating chemical reactions without being consumed in the process. The innovation in nanotechnology and materials chemistry is significantly driven by insights gained from hybridization concepts.
In the realm of organic chemistry, hybridization plays a foundational role in the reactivity of functional groups. For instance, the exposure of sp-hybridized carbons to nucleophiles often leads to unique reaction mechanisms, such as nucleophilic substitutions and eliminations. This chemical behavior underlines the significance of understanding hybridization in designing synthetic pathways for pharmaceuticals and agrochemicals.
In conclusion, hybridization is not merely a foundational concept in chemistry but a gateway to understanding molecular structures and behaviors. The varied types of hybridization—sp, sp², sp³, sp³d, and sp³d²—each contribute uniquely to molecular geometry and properties. From simple hydrocarbons to complex coordination compounds, the implications of hybridization are vast and profound, ranging from theoretical chemistry to practical applications across multiple scientific disciplines. Enhanced comprehension of hybridization thus empowers scientists and chemists to innovate and address pressing challenges in fields from drug development to environmental sustainability.