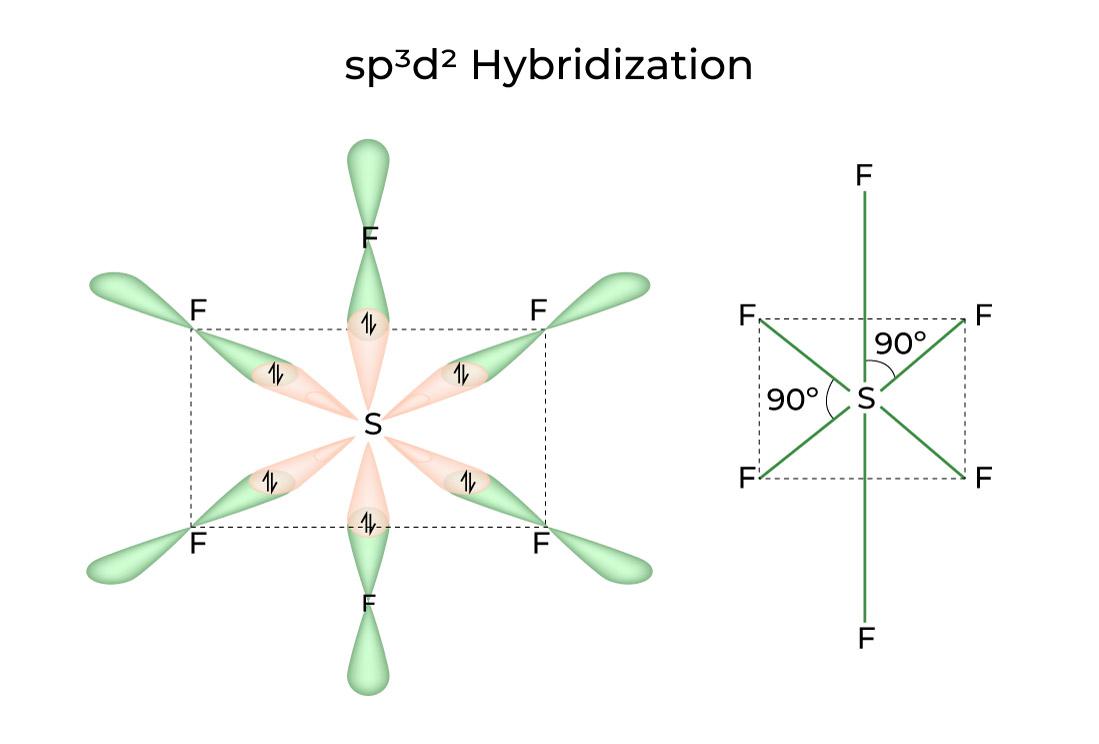
In the ever-expanding universe of chemistry, the hybridization state serves as a foundational concept, akin to the intricate dance of celestial bodies. Just as stars intertwine and move in a harmonious ballet, atomic orbitals—those invisible domains where electrons reside—engage in a similar sort of waltz to form bonds. The process of hybridization, therefore, can be envisioned as the alchemical act of pure elements melding together to create forms more potent than their individual selves.
The essence of hybridization lies in the fusing of atomic orbitals to generate new hybrid orbitals, thus optimizing the spatial arrangement of atoms within a molecule. Consider the carbon atom: within its core, the 2s and three 2p orbitals meld into sp3, sp2, or sp hybrid orbitals, akin to an artist mixing hues to achieve a vibrant palette. This dynamic process allows carbon to form versatile bonding configurations with various elements, propelling it to the forefront of organic chemistry and making it a linchpin of life as we know it.
At the heart of this phenomenon are the different hybridization states, namely sp, sp2, and sp3. Each state offers its own unique geometry and bonding capabilities, contributing to the rich tapestry of molecular architecture.
The sp hybridization is like a slender rocket, aiming for the stars. Here, one s orbital blends with one p orbital, resulting in two degenerate sp hybrid orbitals. This configuration encourages linear geometries at a bond angle of 180 degrees. Prominent examples include acetylene and beryllium chloride, where carbon and beryllium demonstrate this elegant simplicity. The linearity not only signifies efficiency but also minimizes electron repulsion, allowing for a dynamic yet stable molecular structure.
Transitioning from linear to planar structures, we encounter sp2 hybridization, which resembles a sprawling satellite dish, open and inviting. Here, one s orbital combines with two p orbitals to create three sp2 hybrid orbitals, arranged in a trigonal planar configuration around a central atom. The bond angles of approximately 120 degrees reflect an elegant equilibrium that allows molecules like ethylene to thrive. This state enables the fascinating phenomenon of resonance, a defining feature of aromatic compounds, where electrons can delocalize, weaving a complex yet stable tapestry of connectivity.
The sp3 hybridization rounds out the trio, resembling a sturdy tetrahedron, robust and versatile. A hallmark of this state is the formation of four sp3 hybrid orbitals, each spanning 109.5 degrees apart. Methane, with its four hydrogen atoms, epitomizes this configuration, illustrating carbon’s unparalleled ability to form diverse organic structures. The bond angles create a three-dimensional topology that is crucial for the complexity of organic molecules and the intricacies of biological systems.
Delving deeper, one begins to appreciate the interplay between hybridization states and molecular shape in dictating chemical reactivity and properties. The hybridization state is not merely a structural idiosyncrasy; it is a key determinant of the physical and chemical behavior of substances. For instance, the stark contrast between the linearity of a triple bond and the fullness of a tetrahedral bond creates divergent reactivity profiles that inform industrial processes and natural biochemical reactions.
In the grander tapestry of life, hybridization underpins the very essence of molecular evolution. It serves as a bridge, enabling simple compounds to mutate into elaborate biomolecules, setting the stage for life’s complexity. The ability of carbon to hybridize in various configurations is a vital attribute. It offers nature unparalleled flexibility. Whether forming the strands of DNA or the intricate structures of proteins, the hybridization state is fundamental to life’s symphony.
Beyond earthbound applications, hybridization also plays a critical role in fields as varied as materials science and nanotechnology. The manipulation of hybridization allows scientists to engineer materials with tailored properties—from light, flexible polymers to robust, conductive metals. The ramifications of these advancements echo through industries, influencing everything from renewable energy solutions to electronics, further entwining the principles of chemistry with the quest for sustainability.
Moreover, hybridization state influences reactions in organic synthesis. Understanding how various hybridizations dictate reactivity provides chemists with valuable insight into reaction mechanisms, guiding them in designing more efficient synthetic pathways. By mastering the nuances of hybridization, chemists can create molecules that are more effective in catalysis or more potent as pharmaceuticals, extending the reach of chemical science in addressing pressing global challenges—like climate change.
In conclusion, the concept of hybridization state is a rich metaphor for transformation and innovation within the molecular landscape. As we marvel at the myriad forms that life can take, it becomes evident that hybridization is the unseen force that shapes our chemical world into a symphony of possibilities. Understanding it is not simply an academic endeavor; it is a crucial step toward harnessing the potential of chemistry in the fight against climate change, making hybridization not just a technical term, but a beacon of hope and progress in an evolving world.