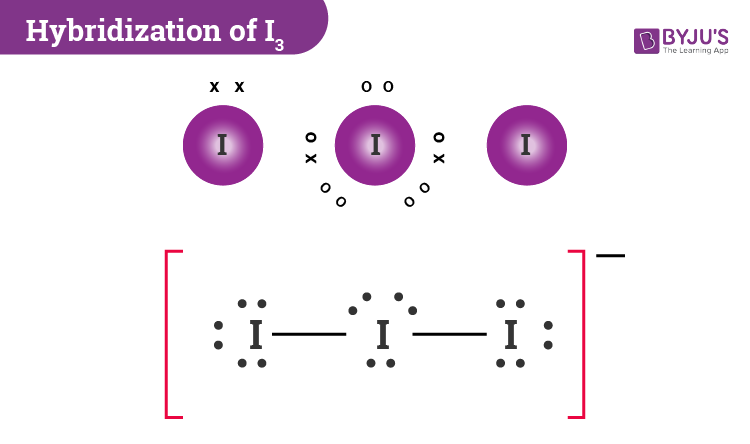
The hybridization of the I3− ion is a captivating dance of electrons, a negotiation between atomic spheres wherein iodine assumes a multifaceted role. Hybridization is a concept that marries atomic orbitals to form new, equivalent bonding orbitals, and in the case of the triiodide ion, this concept becomes an exquisite tapestry showcasing the complexity of chemical interactions.
To grasp the essence of I3− hybridization, we must first delve into its constituent elements. Iodine, an element residing in the halogen group, boasts an atomic number of 53. In its elemental form, iodine presents as a diatomic molecule (I2), where two iodine atoms share electrons. However, the essence of triiodide emerges when iodine molecules encounter potassium iodide or other iodine salts. In doing so, iodine accepts an additional electron, forming the triiodide ion, an entity that carries a negative charge due to the excess electron.
I3− can be likened to an intricate ballet; its structure reveals a trigonal bipyramidal configuration where three iodine atoms are arranged around a central axis. The hybridization of this ion primarily involves the mixing of atomic orbitals, particularly the 5s and 5p orbitals of iodine. In essence, the 5s orbital coalesces with the three 5p orbitals, producing five equivalent sp3d hybrid orbitals. This amalgamation creates a spatial arrangement that optimally minimizes electron pair repulsion, allowing the ion to adopt its characteristic geometry.
At a glance, the appearance of I3− is deceptively simple yet profoundly intricate. The central iodine atom serves as the fulcrum that stabilizes the three peripheral iodine atoms. This configuration is not merely aesthetic; it affects how I3− interacts with other molecules and its overall stability. The geometry, with bond angles approximating 120 degrees between the terminal iodine atoms, ensures that the ion can effectively participate in a range of chemical reactions.
However, the hybridization of I3− is more than just a structural curiosity; it reflects the underlying electronic environment that governs reactivity. The presence of lone pair electrons on iodine creates a rich tapestry of interactions. These lone pairs can influence everything from the stability of I3− in solution to its capacity to form complexes with various metal ions. Thus, the hybridization not only defines the shape of the ion but further informs its chemical behavior as well.
What emerges from this chemical tapestry is a story of balance and dynamism. Each iodine atom contributes to the molecular orchestra, creating a resonance that enhances stability while allowing for reactivity. It is this balance that makes I3− such a significant ion in both inorganic chemistry and various industrial applications. Triiodide is often found in photographic processes, as a reagent in organic synthesis, and even as a food additive. Each of these applications hinges on the ion’s unique hybridization, enabling it to engage in reactions that are pivotal for technological innovation.
Furthermore, the hybridization of I3− opens a window into understanding the concept of resonance structures. The I3− ion exhibits resonance, meaning that multiple valid Lewis structures can represent the same molecule. These structures, while not physically present, offer insight into the distribution of electrons across the ion, thereby enhancing our understanding of its properties. The resonance phenomenon culminates in an electron cloud that is delocalized, which contributes to I3−’s stabilization and versatility as a species.
In broader chemical contexts, hybridization serves as a crucial concept in explaining molecular interactions. The sp3d hybridization seen in I3− is emblematic of the complexities inherent in chemical bonding and molecular geometry. Other species, such as molecules with tetrahedral or octahedral structures, utilize similar principles, showcasing the versatility and adaptation of hybridization across various contexts. Each unique hybridization pattern offers a distinctive insight into the relationship between structure and function. Thus, understanding I3− hybridization empowers chemists to predict the behaviors of numerous molecules and ions.
The implications extend further into the realm of sustainability. In an age characterized by climate change and environmental challenges, understanding chemical processes—including hybridization—can lead to breakthroughs in green chemistry. Innovations in the synthesis and manipulation of such ions have the potential to uncover more sustainable pathways for chemical manufacturing, reducing waste and harmful byproducts. I3−, with its rich electronic characteristics, may hold keys to advancing renewable energy technologies, efficient catalysis, and other green initiatives.
In conclusion, the hybridization of the I3− ion unfolds like a story cast in molecular dynamics, revealing layers of structural elegance, reactivity, and broader implications. Each iodine atom plays a vital role in the ballet of hybridization, demonstrating how detailed atomic arrangements have cascading effects on functionality. From photographic processes to environmental technologies, the I3− ion underscores the sophistication of chemical science, appealing not only to our intellectual curiosity but also to our responsibility as stewards of the environment.