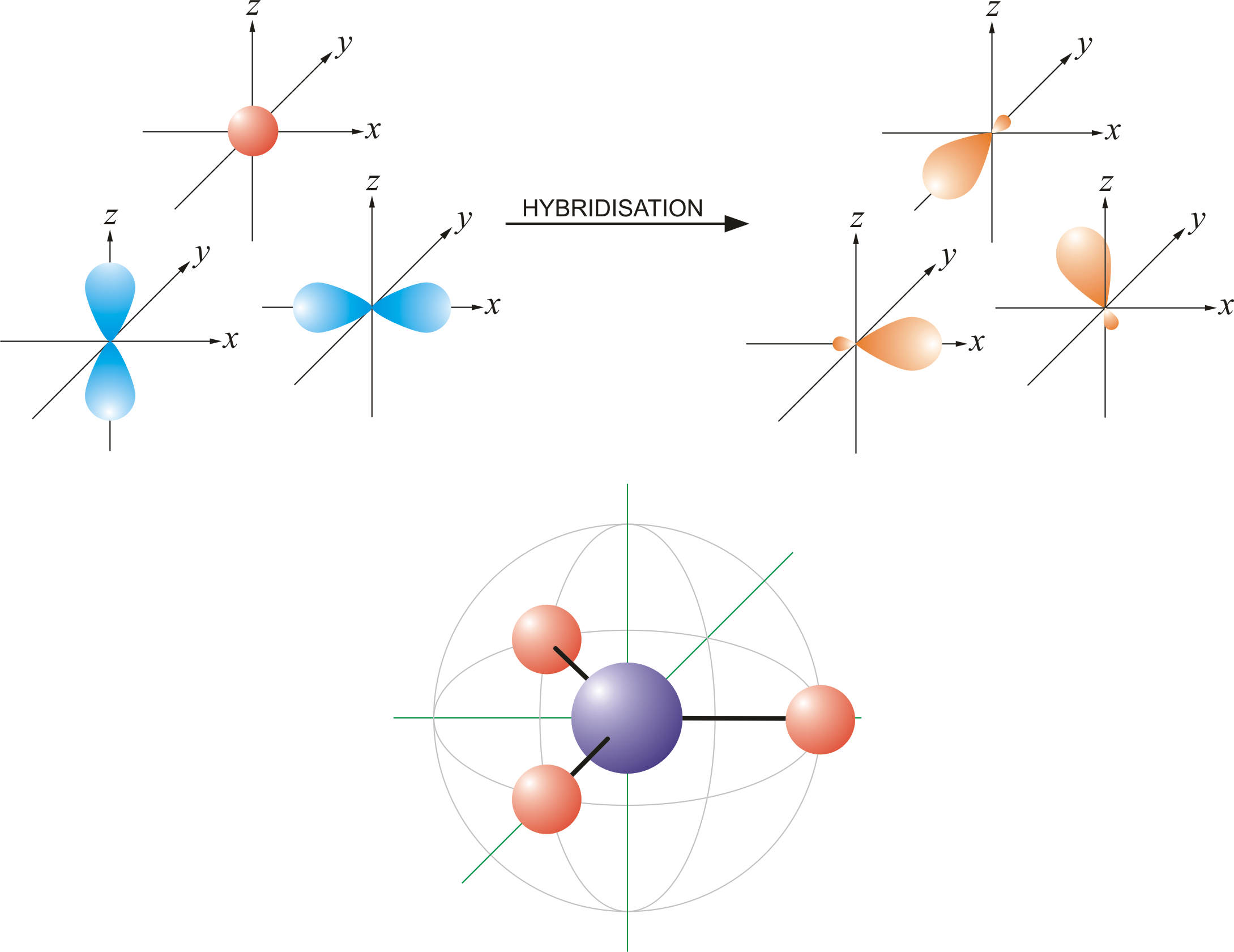
Hybridization is a fundamental concept in chemistry that elucidates the bonding properties of atoms in molecular structures. Among the various hybridizations, sp hybridization plays a vital role in determining molecular geometry, bond angles, and overall reactivity. Understanding sp hybridization necessitates exploring its definition, characteristics, and applications in various chemical compounds.
Definition of sp Hybridization
Sp hybridization occurs when one s orbital and one p orbital combine to form two equivalent sp hybrid orbitals. This process results in a linear arrangement of the orbitals, yielding an angle of 180 degrees between them. The geometrical disposition is particularly conducive for constructing molecules that contain triple bonds or two double bonds.
Characteristics of sp Hybridization
1. Electron Distribution and Geometry: The two sp orbitals are oriented 180 degrees apart, providing a linear geometry. This is characteristic of molecules like acetylene (C₂H₂), where the carbon atoms utilize sp hybridization to form strong triple bonds.
2. Bonding Efficiency: The hybridization allows for efficient overlap between orbitals. This overlapping leads to a strong sigma bond between two bonded atoms. The remaining unhybridized p orbitals can form pi bonds, thereby enhancing the stability and strength of the molecular framework.
3. Examples of sp Hybridization: An array of noteworthy chemicals exemplifies sp hybridization. Ethyne (commonly known as acetylene) serves as one of the most recognizable cases. The carbon atoms in this molecule are sp hybridized, forming one sigma bond and two pi bonds with each other.
Understanding the Bonding in sp Hybridized Compounds
The bonding intricacies of sp hybridized compounds extend beyond mere geometry. In sp hybridization, one can expect a unique bond character differentiating itself from other hybridization types such as sp² and sp³. The resulting bonds tend to exhibit distinct angles and strengths, which are influenced by the contributions of unhybridized p orbitals.
The linear configuration inherent to sp hybridization allows these compounds to achieve maximum stability, especially in compounds with multiple bonds. Each sp hybrid orbital is highly directional, ensuring optimal overlap with adjacent orbitals, whether they be s, p, or other hybridized orbitals.
Applications of sp Hybridization
1. Organic Chemistry: In organic molecules, sp hybridization is often observed in alkynes, where carbon atoms participate in creating linear chains. This directly influences the physical and chemical properties of the substances, such as boiling points, solubility, and reactivity.
2. Inorganics and Coordination Compounds: Metals in coordination complexes can also exhibit sp hybridization, where it plays a pivotal role in forming stable complexes. Transition metals, for example, utilize hybridization to engage with ligands in defining their coordination number and geometry.
3. Material Science: Advances in material science often incorporate sp hybridized materials for their unique electronic properties. Carbon-based materials like graphene possess sp hybridization, granting remarkable electrical conductivity and strength, useful in various electronic devices and composite materials.
Contrasting sp Hybridization with Other Types
To fully appreciate sp hybridization, it is essential to contrast it with sp² and sp³ hybridizations. While sp hybridization lets atoms arrange themselves linearly, sp² hybridization involves one s orbital and two p orbitals, resulting in three sp² hybrid orbitals that form trigonal planar structures with bond angles of 120 degrees. Contrastingly, sp³ hybridization encompasses four equivalent orbitals, leading to a tetrahedral shape with bond angles of 109.5 degrees.
This divergence in hybridization affects molecular properties significantly. For instance, sp hybridized molecules are typically less reactive than their sp² and sp³ counterparts owing to the robustness of the triple bond formation. The linear shaped molecules exhibit distinctive characteristics in reaction pathways, influencing their chemical behavior.
Summary and Conclusion
In conclusion, sp hybridization stands as a pivotal concept that elucidates the structure and reactivity of numerous chemical compounds. Understanding its characteristics, applications, and contrasts with other hybridization types enables chemists and geological scientists to make informed predictions about molecular behavior. Whether in organic chemistry, inorganic complexes, or materials development, sp hybridization plays a critical role in shaping the molecular landscapes of our world.
As climate change and environmental degradation become increasingly urgent issues, comprehending the chemical properties that underpin various compounds—many of which are influenced by hybridization—will be crucial. This ingrained understanding not only informs cleaner, greener practices in chemistry but also contributes to fostering an environmentally sustainable future.