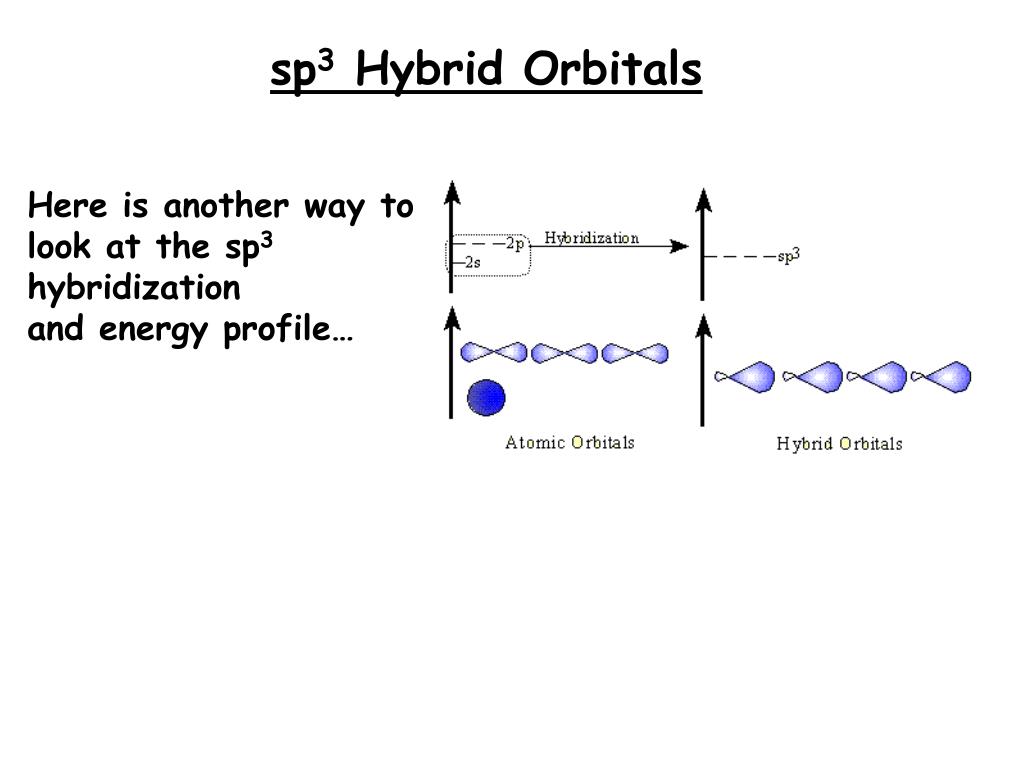
Hybridization is a fundamental concept in chemistry that elucidates the formation of molecular structures through the combination of atomic orbitals. The sp3 hybridization is particularly significant due to its prevalence in organic molecules and its role in determining molecular geometry. Understanding sp3 hybridization involves comprehending the characteristics of hybrid orbitals, their three-dimensional shapes, and the types of molecules they help to form.
At its core, sp3 hybridization occurs when one s orbital and three p orbitals from the valence shell of an atom amalgamate to create four equivalent hybrid orbitals. These hybrid orbitals exhibit unique geometric properties, enabling atoms, particularly carbon, to form stable bonds by sharing electron pairs with adjacent atoms.
The geometry of sp3 hybridization is tetrahedral, a term derived from the Greek word “tetra,” meaning four. In a tetrahedral configuration, the four sp3 hybrid orbitals are oriented in three-dimensional space at angles of approximately 109.5 degrees relative to one another. This spatial arrangement minimizes electron pair repulsion (as described by VSEPR theory), thereby stabilizing the molecular structure.
Consider ethane (C2H6), a quintessential example of sp3 hybridization. Each carbon atom in ethane undergoes sp3 hybridization, resulting in the formation of four equivalent sp3 hybrid orbitals. These orbitals extend outward, forming sigma bonds with the three hydrogen atoms and another carbon atom. The resulting molecular structure showcases the tetrahedral spatial arrangement, characterized by robust single bonds.
Another noteworthy aspect of sp3 hybridization is its manifestation in other molecules, such as methane (CH4). In methane, the central carbon atom undergoes sp3 hybridization, leading to the formation of four sigma bonds with hydrogen. The tetrahedral configuration results in a symmetrical distribution of electron density around the carbon, contributing to methane’s stability. In fact, methane is the simplest alkane and serves as a reference point for understanding larger hydrocarbons.
Beyond hydrocarbons, sp3 hybridization is prevalent in various functional groups, including alcohols and amines. For instance, in ethanol (C2H53 hybridization, ensuring stable bonding with the hydroxyl (-OH) group. Similarly, in amines, nitrogen adopts an sp3 hybridization, allowing it to bond with carbon or hydrogen atoms while accommodating the lone pair of electrons. Consequently, the tetrahedral configuration influences the physical and chemical properties of these organic compounds.
The ability to form strong covalent bonds is one of the most critical advantages of sp3 hybridization. The overlapping of hybrid orbitals leads to the formation of sigma bonds, which are generally stronger than pi bonds. This results in stable and durable molecular structures that can maintain their integrity under various environmental conditions. The stability is further enhanced by the saturation of carbon in these molecules, reducing the likelihood of reactivity.
However, sp3 hybridization goes beyond mere bond formation. The geometrical implications of sp3 hybridization also extend to the stereochemistry of molecules. The arrangement of substituents around a tetrahedral carbon can lead to specific spatial configurations, including chirality. Chirality is a crucial consideration in biochemistry and pharmaceuticals, where the spatial orientation of molecules can significantly impact biological interactions and drug efficacy.
Furthermore, sp3 hybridized compounds often exhibit unique physical properties, such as boiling and melting points, which are influenced by the intermolecular forces present in the substances. For instance, the presence of dipole-dipole interactions in polar sp3 hybridized molecules like alcohols can affect their boiling points, distinguishing them from their nonpolar counterparts. Consequently, understanding sp3 hybridization is essential for predicting and controlling the physical and chemical behaviors of various organic compounds.
In summary, the concept of sp3 hybridization is integral to the study of molecular chemistry. It provides a framework for grasping how atoms bond, how molecular shapes are determined, and how these shapes influence the properties of substances. From the ubiquitous methane to complex organic molecules containing functional groups, sp3 hybridization plays a pivotal role in defining molecular architecture.
Ultimately, the exploration of sp3 hybridization not only enriches our understanding of molecular structures but also enhances our ability to design and synthesize new compounds with desirable properties. Consequently, such knowledge holds paramount importance in fields ranging from pharmaceuticals to materials science. In this age of scientific advancement, the knowledge of hybridization and its implications can empower researchers and innovators to tackle challenges across diverse disciplines.