The concept of sp3d hybridization presents an intriguing nexus between atomic theory and molecular structure, specifically within the realm of trigonal bipyramidal geometries. This advanced hybridization mode, although less commonly encountered than sp3 or sp2, brings a wealth of understanding to complex molecular forms. So, what exactly is sp3d hybridization, and how does it influence molecular geometry?
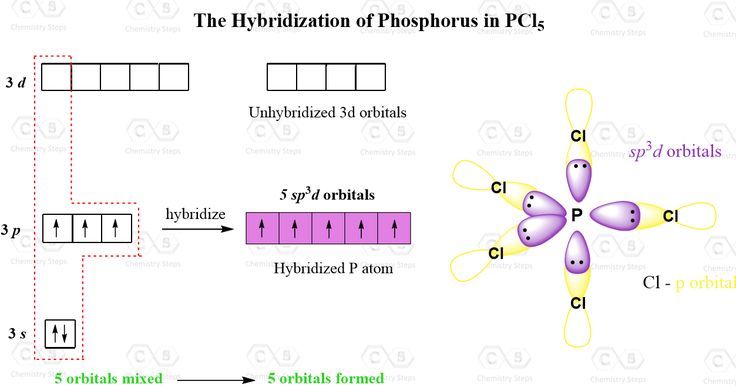
To comprehend sp3d hybridization, one must first grasp the fundamental principles of atomic orbitals. In essence, atomic orbitals are regions in an atom where the probability of finding electrons is maximized. These orbitals include s, p, and d types, each with distinctive shapes and orientations. The combination of one s, three p, and one d orbital leads to the unique configuration representative of sp3d hybridization.
In sp3d hybridization, the atomic orbitals merge to create five equivalent sp3d hybrid orbitals. These orbitals arrange themselves in a trigonal bipyramidal geometry. This geometry consists of two distinct positions: three orbitals occupy an equatorial plane while two reside axially. This configuration is crucial for understanding the electronic structure of certain molecules, particularly those that have five substituents or electron clouds around a central atom.
A classic example of a molecule exhibiting sp3d hybridization is phosphorus pentachloride (PCl5). In this compound, phosphorus serves as the central atom, surrounded by five chlorine atoms. The hybridization of phosphorus enables the formation of strong covalent bonds with chlorine, resulting in a stable compound. The trigonal bipyramidal arrangement of the chlorine atoms minimizes electron pair repulsion, in accordance with VSEPR (Valence Shell Electron Pair Repulsion) theory, thus enhancing molecular stability.
The significance of sp3d hybridization extends beyond mere molecular bonding. Understanding this hybridization gives insight into the physical and chemical properties of various compounds. For instance, the geometry inherent to sp3d hybridization leads to unique dipole moments in molecules. This is particularly discernible in transition metal complexes, where the d orbitals play a pivotal role in influencing coordination chemistry and reactivity.
Furthermore, sp3d hybridization has implications in the field of materials science. In designing catalysts for industrial reactions, molecular structure often dictates efficiency. Consequently, tailored hybridization could lead to breakthroughs in sustainable practices, particularly in reducing greenhouse gas emissions. Scientists hypothesize whether modifying hybridization might yield more effective catalysts, a playful inquiry that illustrates the challenge of marrying theoretical chemistry with practical environmental solutions.
In the realm of molecular interactions, sp3d hybridization can also be observed in various transition metal complexes, which are crucial in biological and synthetic systems alike. These complexes often exhibit properties such as color, magnetism, and reactivity derived from the arrangement of d electrons in relation to the hybrid orbitals. The exploration of these properties opens avenues for innovation in drug design and bioinorganic chemistry, further underscoring the importance of hybridization in a multidisciplinary context.
However, what happens when trigonal bipyramidal geometry faces real-world constraints? There lies a potential challenge in ensuring that synthesized compounds retain their intended hybridization. Complex environments, such as those found in biological systems or industrial applications, can alter the stability of hybrid orbitals. This dynamic aspect of chemistry poses an essential question: can we predict and control hybridization under varying conditions?
The pursuit of understanding sp3d hybridization is not merely an academic exercise; it resonates with practical implications that may influence future advancements in technology and environmental sustainability. For example, hybridization could be manipulated to optimize solar cells or improve catalysts for green chemistry processes. Hence, ongoing research into molecular structures and their hybridization states is crucial in addressing contemporary challenges in energy efficiency and reducing the carbon footprint.
The exploration of enclosed geometric configurations also serves as an intellectual exercise. The unpredictability of molecular interactions challenges scientists to think critically about design and implementation. For instance, could there be a way to harness the properties of sp3d hybridized molecules to create novel materials that address climate change? Such inquiries would necessitate a balance between chemistry and environmental science, echoing the interdisciplinary nature of modern research.
In conclusion, sp3d hybridization encapsulates a fundamental aspect of molecular chemistry that intertwines with broader themes of sustainability and innovation. By examining the intricacies of hybridization, one not only benefits from understanding molecular structures but also opens avenues for addressing urgent global challenges. As the scientific community continues to probe these complex relationships, the potential for transformative solutions remains as enticing as it is necessary, beckoning a new generation of scientists to ponder the fusion of hybridization and environmental stewardship in the realm of modern chemistry.